The FDA and Lupin Pharmaceuticals recently announced a voluntary recall for certain blood-pressure tablets because they might contain cancer-causing ingredients.
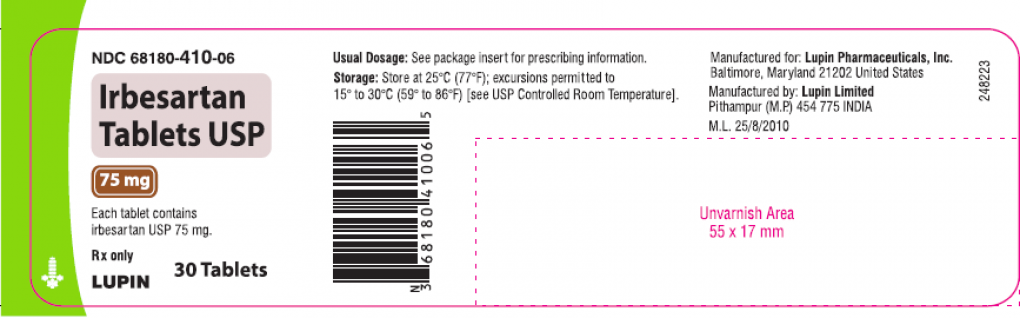
An urgent recall was issued earlier this week for two blood-pressure drugs that may contain ingredients that might cause cancer, according to the U.S. Food and Drug Administration (FDA). The voluntary recall was issued by Lupin Pharmaceuticals and includes all of the company’s “Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets.”

The recall was issued soon after the affected tablets underwent testing that “revealed that several batches of both medications, which are used to lower blood pressure, exceeded acceptable levels of the impurity N-nitrosoirbesartan, a probable human carcinogen—meaning it could cause cancer, based on laboratory testing,” the FDA notes. In fact, according to the FDA, “more than 90% of the over 300 known N-nitroso compounds (one of which is N-nitrosoirbesartan) were found to be carcinogenic when tested on animals.”
The recall notice further states:
“Out of an abundance of caution, Lupin is recalling all batches of Irbesartan tablets (including 75mg, 150mg and300 mg sizes in 30- and 90-count bottles) and all batches of the drug Irbesartan and Hydrochlorothiazide tablets (including 150mg/12.5mg and 300mg/12.5mg sizes in 30- and 90-count bottles) in the United States.”
A full list of the recalled products can be found here.
Consumers who have the recalled tablets should contact their doctor for an alternative treatment. It is not advised to suddenly stop taking the tablets at the time because doing so without an alternative treatment plan could cause adverse health issues. So far, Lupin has received four reports of consumers falling ill from Irbesartan. Fortunately, there have been no similar reports linked to the Irbesartan and Hydrochlorothiazide tablet.
If you have additional questions or concerns about the recall, contact 855-769-3988 or 855-769-3989.

Join the conversation!