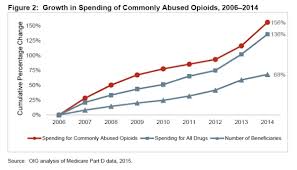
HHS Reports identify Rampant Medicare Part D Fraud
6/24/2015 Two reports released on Tuesday highlight the persistence of Medicare fraud, especially involving the Part D prescription drug program. The reports follow the largest Healthcare Fraud takedown in history last week, as officials charged 46 doctors and pharmacy owners, as well as nearly 200 others for Medicare fraud. This includes the arrest of 44