The CPSC recently announced a recall for certain bottles of pain relievers that don’t meet child-resistant packaging requirements.
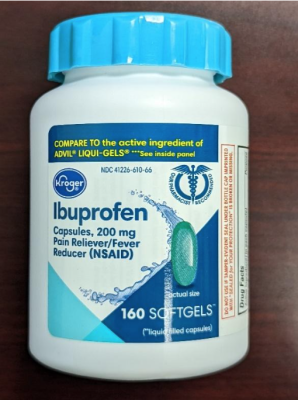
Earlier this week, an urgent recall was issued for more than 407,000 bottles of popular pain relievers, including acetaminophen, ibuprofen, and aspirin. According to the Consumer Product Safety Commission (CPSC), the bottles were recalled “for not meeting child-resistant packaging requirements.”
The recall is nationwide, though it’s important to note that there is nothing wrong with the actual medication. The issue is that because the bottles “don’t meet standards for child resistance…a child could open them and accidentally ingest the medications inside.”
The affected products are all sold under the Kroger and Walgreens brand and include the following products:
- 34,660 Kroger Brand Acetaminophen, 100 count bottles
- 25,660 Kroger Arthritis Pain Acetaminophen, 225 count bottles
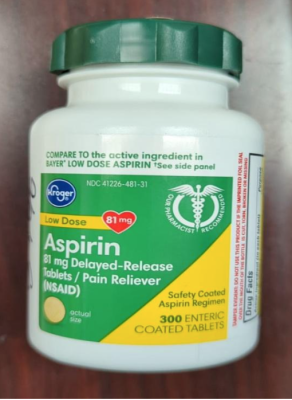
- 209,430 Kroger Aspirin, 300 count bottles, and Ibuprofen, 160 count bottles
- 137,300 Walgreens Pain Reliever Acetaminophen, 150 count bottles
Fortunately, no injuries have been reported in connection to the recall. For now, the CPSC is advising consumers to store the recalled products in a safe spot where children cannot reach them. Additionally, consumers can contact Aurohealth or Kroger for instructions on how to return or dispose of the product.
Sources:
Recall: More than 400K pill bottles recalled for not meeting child-resistant packaging requirements

Join the conversation!