A voluntary recall was issued earlier this week for certain types of thyroid medication that may not be potent enough to be effective.

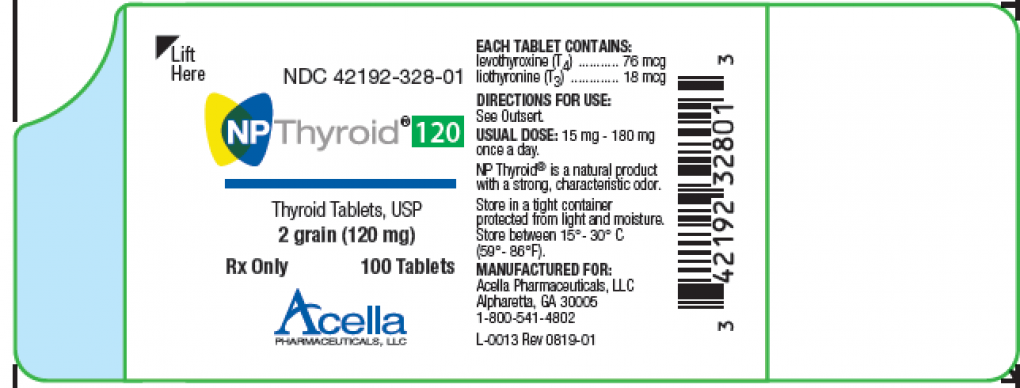
Earlier this week, a voluntary recall was issued for a couple of types of thyroid medication that “may not be strong enough to be effective,” according to a notice posted by the FDA. The manufacturer of the medication, Acella Pharmaceuticals, stated “one lot of 15-mg and one lot of 120-mg NP Thyroid, Thyroid Tablets, USP were found to be sub potent.”
According to the notice, the affected medications are used to help treat “an underactive thyroid.” They’re packaged in 100-count bottles and expire between October 2020 and November 2020. The products have the following lot numbers: M327E19-1 and M328F19-3. If you or someone you know are taking one of the recalled medications, you should contact your healthcare provider immediately before you stop taking it. Stopping certain medications cold turkey may pose unintended health risks. Underactive thyroids are serious and require medical attention to help regulate them. When left unchecked, underactive thyroids can cause symptoms like “swelling, depression, and a slow heart rate.”
If you took the recalled medication and experienced adverse reactions, contact the FDA’s MedWatch Adverse Event Reporting program at 1- 800-332-1088.
Sources:
Thyroid Medications Recalled Because They May Not Be Strong Enough

Join the conversation!