Medtronic is recalling certain HawkOne devices that may cause patient injury.
Earlier this week, Medtronic announced a Class I medical device recall for its HawkOne system designed for clearing out clogged arteries. The recall was issued after the company received numerous injury reports. So far, more than 95,000 devices distributed throughout the U.S. since 2018 are included in the recall.
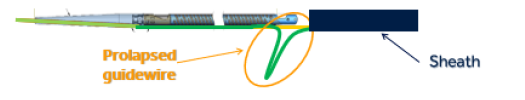
The HawkOne system is designed with a catheter and a “powered cutter that is designed to strip away plaque build-up inside peripheral arteries by spinning at up to 12,000 RPM.” The issue that prompted the recall has to do with the “device’s guidewire, which can become bent and stick outwards during use.” When this happens, the catheter tip might separate or break off, which could result in “serious complications, including a ruptured or blocked blood vessel, which would require surgery to repair the artery and retrieve the device.”
So far, the FDA has received “163 complaints about the device, linked to 55 injuries and zero deaths.” When asked what patients should do for the time being, Medtronic noted that “no action is required for patients who underwent procedures without incident, and current patients should be monitored per the practice’s normal follow-up procedures.”
If you have additional questions or concerns, contact your Medtronic Field Representative or call Medtronic Customer Service at (800) 854-3570.
Sources:
Medtronic snags another Class I recall, this time for its artery-clearing HawkOne system
Medtronic Recalls HawkOne Atherectomy System Due to Risk of Tip Damage During Use


Join the conversation!